In this article, I will provide an overview of my take on the current zeitgeist of reefing and explain why the entire industry is ignoring a critical aspect of coral care. I will explain what issues this parameter causes, how to monitor and test for it, and how to manage it in your system. Ultimately, I will synthesize this information to form the basis of a new reefing method.
The Beginning of the End
At the time of writing this article, I am in the Bahamas. I have been excited to come here and see the reefs for months. Early this week, the day finally came. After lunch, I approached the beach, where I could observe dark structures beneath the waves around 100 feet off the shore. I donned my mask and snorkel and began to swim.
My mind was racing about what species I may get to observe in their natural habitat – Porites? Acropora palmata? Finally, a sandy bottom turned into a rocky outcropping. I cleared my mask and was met with a sight that crushed me. Instead of fields of coral as I expected, I was met with… algae: macroalgae, sponges, and death.
The reefs were no more – at least not as they used to be. The few corals I did see all had visible symptoms of diseases I had only read about until that point – brown band disease, stony coral tissue loss disease, what looked like fungal infections characterized by dark purple lesions in gorgonians and sea fans. The reefs were devastated.
I sat there floating – reflecting on what I was witnessing. At that moment, I realized that until then, some of me still had hope the reports were over-sensationalized – that maybe I would get lucky and find a pristine reef on the least populated island in the Bahamas. I learned that day for the reefs – luck had run out.
For the remainder of the day, I was distraught. I spent much time outside, walking along the tide pools and thinking. Eventually, I came to terms with the grim reality of the situation. I determined that while I could personally do nothing to save these reefs, what I could do was learn about what caused their demise and apply that knowledge in a positive way somewhere else. In the past, I had read much about phase shifts where reefs would move from coral to algae or sponge dominant. I then remembered a trail of research I had started down but never picked back up – the work of Dr. Forest Rohwer.
Dr. Rohwer is a microbial ecologist at San Diego University. He was the first person to use shotgun metagenomics in 2002 and helped open the door to using omics techniques in modern science. He and Dr. Nancy Knowlton founded the holobiont theory of corals and were the first to propose that corals were metaorganisms. In addition to these significant breakthroughs, he helped develop the DDAM model of reef recession (which I will discuss in depth) and the Piggyback-the-Winner model of bacteriophage regulation (which I will discuss in a future article).
After reviewing Dr. Rowher and his colleague’s work, I have gleaned much that I think could drastically improve the care of our animals. In the remainder of this article, I will summarize my findings, reflect on our current methods, and extrapolate where I think we can improve the care we give our corals.

The DDAM Model
After nearly a decade of in situ and ex-situ research with data points from over 60 reefs, the DDAM model holds weight as the most accurate explanation for the decline of reefs. DDAM stands for dissolved organic carbon (DOC), disease, algae, and microorganism. This model posits that higher pollution rates have led to a sharp increase in DOC on reefs.
This increase in DOC combined with the negative effect overfishing has on herbivore populations lead to excess algae growth. Algae directly compete with coral for space. They produce allelopathic compounds that inhibit coral growth and larval settlement and create even more DOC as a byproduct of their metabolism.
Writ large, the reef shifts from an oligotrophic (low carbon) to a copeotrophic (high carbon) environment. This shift in DOC has a direct impact on the microbial ecosystem. High levels of DOC have been proven to correlate with virulent gene expression in potential pathogens. High DOC also shifts the holobiont to a dysbiotic state by allowing r strategy opportunists to thrive over endemic regulatory symbionts. [1]
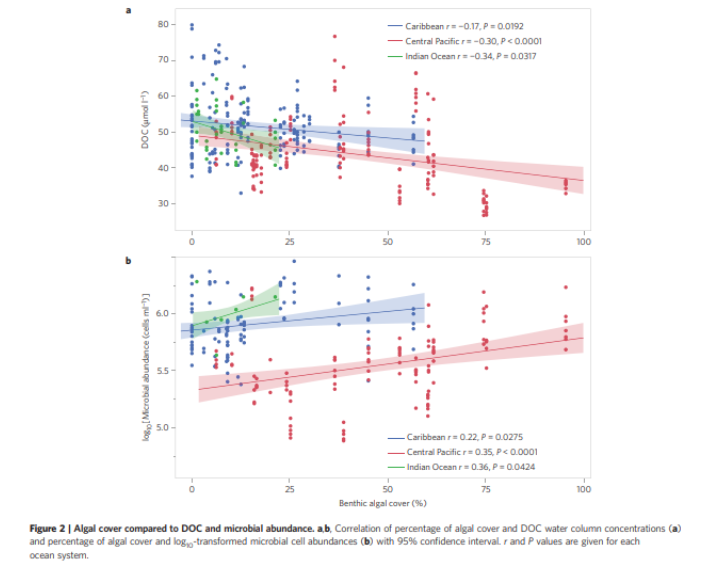
In short, healthy reefs are environments with low nutrients and DOC and high concentrations of herbivorous fish. This allows for the effective management of algae leading to high coral cover at the macro level. At the micro level, this oligotrophic environment allows picoplankton in the water column and regulatory families of bacteria in the holobiont to thrive. Those bacterial families are critical to the coral’s health, providing both external nutrition and holding together the structure of the metaorganism.
High values of DOC cause them to become outcompeted by harmful bacteria, which then causes coral to die from disease. As more corals die, there’s more room for algae to colonize, reinforcing the cycle. This is exacerbated further via climate change, as temperature and pH swings can act as sources of additional abiotic stress, which causes coral bleaching or die-off. [2]
Application to Aquaria
I’ll first dive into some applied microbiology to understand the effect of high DOC levels on our system. Two primary pools of bacteria affect corals. The external planktonic bacteria in the water column and the bacterial symbionts that are directly associated with the coral. Both population groups are fluid based on biotic and abiotic factors in the environment.
Coral disease is complex to solve because many commensal bacterial species that play essential roles in nutrient cycling or antibiotic production in a healthy ecosystem can become opportunistic pathogens once the external environment changes.
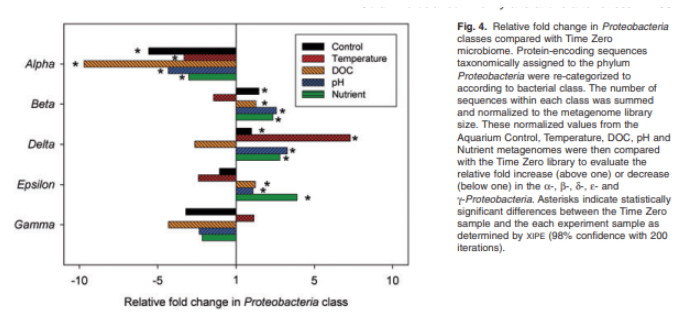
A large study led by Dr. Rebecca Vega Thurber took corals from the wild and exposed them to temperature, pH, nutrient, and DOC shifts in a controlled environment. The microbiome and transcriptome were tracked during these chemical changes to reveal how the microbial world’s interaction with the corals shifted in a chaotic environment.[3]
We can apply several of the primary findings of this study to the industry. First, it was found that the diversity of the holobiont narrowed after corals were introduced to aquariums. In nature, the availability of potential symbionts in the environment and the unstable nature of oceanic chemistry leads to a constant shuffling of the microbial members of the holobiont as different species interact with the system and affect its dynamics.
In an aquarium, since there’s more stability and much less heterogeneity in the bacterial population, the holobiont differentiates and stabilizes from its wild type to better suit aquarium life. Over time, the potential symbionts that fail to associate with the coral get outcompeted as other species settle into their niches. This leads to a loss of microbial biodiversity – ultimately, fewer bacteria can live in captivity than in the ocean.
Second, it’s shown that there are distinct shifts in microbial taxa and gene expression depending on the abiotic stressor. Different bacterial issues will manifest if you have a pH swing vs a temperature swing. Different species can capitalize on different environmental shifts, leading to distinct microbial profiles after a stress event.
This further complicates the diagnosis and treatment of coral disease since even if corals express the same external symptoms (tissue necrosis, etc.), there could be a multitude of pathways through which those symptoms could have arisen; thus, it may be essential to understand the total life history of corals and what stressors they’ve been exposed to before any treatment.
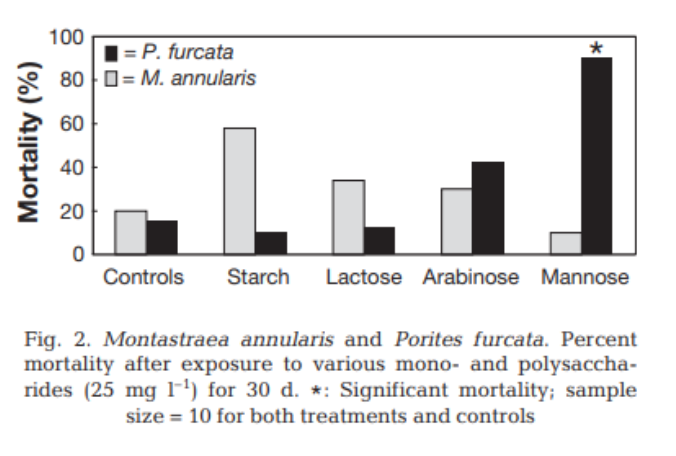
While the Thurber study examines the overall trends associated with chemical swings, it’s important to contextualize the microbiome specifically to the effects of high DOC. I found another paper that analyzed labile DOC’s direct effect on the virulence factors of coral-associated bacterioplankton.
This study found that healthy reef environments are oligotrophic (low nutrient) and typically have a higher concentration of Alphaproteobacteria (Pelagibacter are members of this class), and unhealthy reefs are copiotrophic (high nutrient) and have high concentrations of Gammaproteobacteria (vibrio are a member of this class).
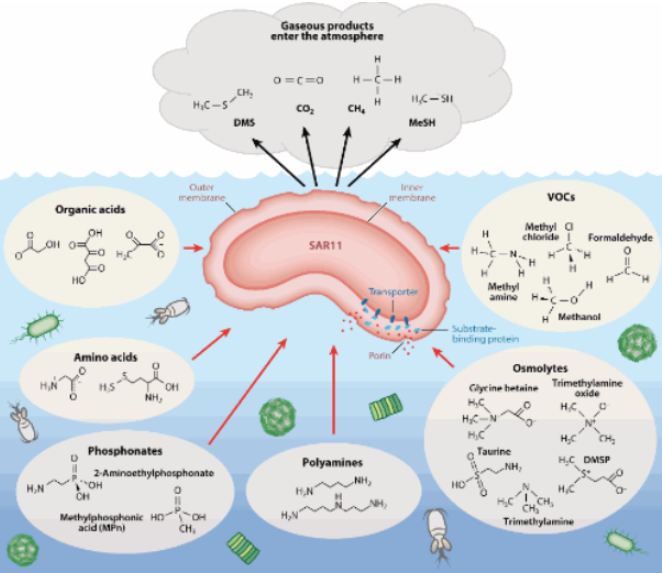
It was revealed that the sugars glucose and mannose were the primary carbon sources that led to harmful microbial shifts. Mechanistically, many of the beneficial picoplankton species, such as those in the SAR11 order or potentially important coral symbionts such as Endozoicomonas that live an oligotrophic lifestyle likely “have a smaller number of broad-specificity and sufficiently high-affinity transporters” while potential pathogens that lead a copiotrophic lifestyle “possess a wide array of low-affinity and highly specific transporters”[4]
Additionally, many potential pathogens had low-affinity sugar uptake transport systems, such as ABC systems and those belonging to the multi-sugar facilitatory superfamily. Because copiotrophs have many different sugar uptake strategies, they can outcompete bacteria that have evolved in oligotrophic conditions when there are high levels of environmental DOC.
It was also found that when these alternate sugar metabolism pathways are expressed, many virulence factors are upregulated, including biofilm production, siderophores, toxins, and the expression of metalloproteases for iron acquisition. It was found that the primary species that exhibited this pathogenic shift were existing symbionts associated with coral. Additionally, these bacterial taxa never comprised more than 4{4213dd704bcac8bc0f4b7ed263c80e178fe24d9d0d3162a98d050d1d839166e8} of the sample population; thus, they are classified as “rare taxa.”
In layman’s terms, whenever water on a reef becomes polluted with DOC, a small amount of “sleeper cell” bacteria that typically perform beneficial tasks for the coral become activated like a Manchurian candidate and begin to wreak havoc on the corals, leading to the development of disease. i.e., dirty water = dirty bacteria.
These principles likely hold true in our reef tanks. Regarding bacterioplankton, numerous papers have illustrated that corals selectively uptake three primary groups of bacteria as food: Cyanobacteria Procholorococus and Synechococcus and members of the SAR11 order.
When reviewing the database from Aquabiomics, the microbial community in our tanks isn’t identical to nature, and the primary picoplankton species we find in our systems are those of the SAR11 order (Pelagibacteracea). This is likely an effect of the microbial homogenization discussed in the Thurber paper, where the microbiome of marine systems is likely to decrease in diversity in closed systems.[5]
Because the primary species of beneficial bacterioplankton that persist in our system are Pelagibacteracea we need to understand the SAR11 order to know how this affects our system. Pelagibacter are elementary organisms that have been on this planet since some of the first life. These species have evolved to metabolize exclusively low molecular weight DOC and often get rapidly out-competed in environments with high DOC values.
Based on anecdotal results viewing the microbiomes of many systems, the most successful tanks tend to have a sizable dominant band of Pelagibacteracea in their reports. This would theoretically help structure the planktonic community and give our corals nutrition.
Typically, when the environment in our tanks doesn’t favor these species, people tend to see broader bands of families that are more likely to be pathogenic, such as Vibrio, Rhodobacteracea, or Altermonadacea. As discussed above, Pelagibacter thrive in low DOC environments; thus, they’re likely to be outcompeted in systems with high levels of DOC.[6]
This is an essential concept for the many people attempting to get broad bands of Pelagibacter in their system. Even if bottled Pelagibacter was on the market, if the environment of a system does not facilitate its growth, it doesn’t matter how much of it you could theoretically add – if it doesn’t have the correct environment to gain a foothold and colonize then it will not persist in your system. Thus, a prerequisite for a balanced microbiome is an oligotrophic environment – this can only be achieved by having low DOC values.
This also explains why so many people who keep their inorganic chemistry in perfect order are still experiencing issues with coral disease. Because many trace element programs require users to stop water changes, this would allow for the continual build-up of DOC. Therefore, even if all trace elements come back as perfect on an ICP test and all parameters are stable, chronic exposure to high levels of DOC will always trigger a pathogenic shift.
Now that we understand how high DOC can affect our systems (high rates of coral disease due to an unbalanced microbiome) let’s identify where DOC could originate in our systems.
1. Food: Any food added to a system will eventually break down and become part of the organic carbon pool – this includes coral and fish foods, frozen and powdered, freeze-dried, and flakes. All food is fundamentally a combination of complex carbon sources.
2. Amino Acids: Almost everyone I know adds amino acids to their systems. Due to their short half-life in aqueous environments, they are likely one of the primary candidates for contributing to high DOC levels, especially if you utilize them on a doser and add them daily.
3. Carbon Dosing: Adding any carbon source will lead to a direct increase in organic carbon concentrations.
4. Refugiums: It’s well established in the scientific literature that almost all macroalgae species produce copious amounts of excess DOC as established by the DDAM model – it’s likely the algae species we use for inorganic nutrient export are a double-edged sword that also increases our organic nutrient values.
5. No Water Changes: Recent trends with ICP and trace element management have led to people discontinuing water changes on their systems – this likely leads to systems that utilize these approaches having higher DOC values on average
How Can DOC be Managed?
There are various traditional methods through which DOC can be removed from our system.
1. Protein Skimmers: these aid in removing some portion of the DOC in our tanks. [7]
2. Ozone: Many peer-reviewed articles have shown that in the context of wastewater treatment, ozone can convert some organic compounds into aromatic proteins, which other filtration methods can then remove.
3. GAC: There’s lots of data that shows activated carbon can bind to some compounds that make up DOC and remove it from a system. [8]
4. Water Changes: Dilution is often the best and only solution.
While these have all been touted as typical solutions to lowering DOC in the past, they have some cons worth considering. Protein skimmers only solve part of the problem and can never unilaterally remove all of the DOC in a system due to the nature of bubble dynamics. Ozone likely has a very negative effect on the microbiome.
There’s mounting evidence that ozone may contribute to eliminating Pelagibacteracea in our systems, which can shift the microbial community to an unbalanced state. GAC seems to be the most promising low-work approach, but it still doesn’t remove all the DOC pool’s chemical species. Additionally, it may bind to some beneficial trace elements and there is an ongoing discussion about its potential role in contributing to the development of HLLE .
Water changes can become expensive, take time, and derail many of the new trace element programs that many subscribe to today.
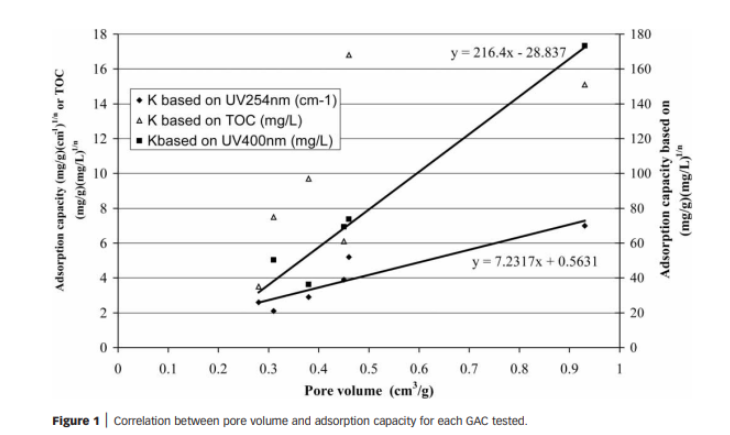
Given that these approaches (besides water changes) solve part of the problem, GAC and ozone have potentially significant adverse effects (these need to be tested), and water changes are increasingly falling out of favor. Is there an option to remove DOC that is easy and doesn’t harm the system?
Enter Our Savior: Sponges
After some brainstorming and a deep dive into the literature, the most logical answer that comes to my mind is to utilize an organism to solve the issue. This would allow for a low-cost, low-maintenance approach that meshes well with current reefing methodology that’s steering away from water changes. It also likely holds the keys to structuring the microbiome in a beneficial way.
In nature, sponges complete the carbon cycle by uptaking massive amounts of DOC and then converting that dissolved carbon into particulate organic carbon (POC), which is shed into the water column and eventually acts as food for corals. On healthy reefs, “sponge looping” effectively recycles excess DOC into additional food for the corals on top of the picoplankton they’re already consuming.
One controlled study analyzed the rate at which three species of cryptic sponges found in the Caribbean uptook DOC. It was found that carbon fluxes averaged “14.3-18.5 µmol C cm-3 sponge h-1.”. Assuming 12 hours of daily activity, this would amount to a carbon flux of “5.15-6.66 g C m-2 sponge d-1.”. It was also found that 90{4213dd704bcac8bc0f4b7ed263c80e178fe24d9d0d3162a98d050d1d839166e8} of the total carbon uptaken by sponges was from DOC, not alternative carbon sources such as live bacterioplankton.
Additionally, incubation experiments compared the DOC clearance rates between sponge + sediment communities and calcareous algae + bare substrate communities. The results from these experiments illustrated that the sponge-covered substrate removed “almost 1 mol C m-2 d-1,” and the typical reef surface did not affect aqueous carbon values. This highlights that cryptic communities, in particular, play a key role in carbon cycling compared to other reef surfaces and emphasizes the importance of creating such spaces in our systems. [9]
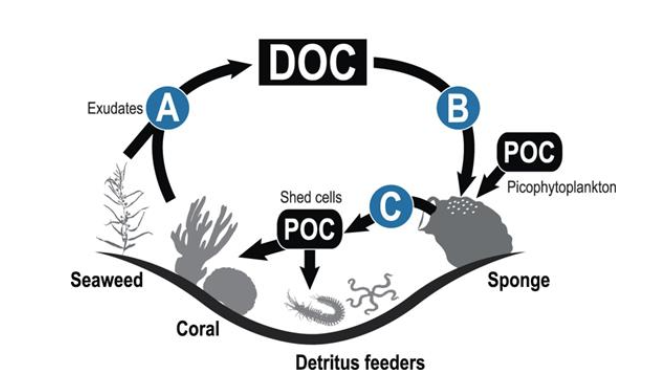
Another critical aspect of sponges is their ability to house many beneficial bacterial partners. Some partners are excellent at sequestering phosphate. A recent study illustrated that nearly 40{4213dd704bcac8bc0f4b7ed263c80e178fe24d9d0d3162a98d050d1d839166e8} of the phosphate found in sponges was contained in granules composed of polyphosphate. This mineralization allows waterborne phosphate to be locked away and removed from a system. This could theoretically help decrease PO4 in a system.
Additionally, many species of nitrifying bacteria call sponges home, including anaerobic bacteria capable of anammox. Annamox is a chemical process of ammonia oxidation where bacteria can convert ammonia to nitrite and then nitrite to nitrogen gas. This skips the typical nitrate conversion step and allows nitrogen to leave the aqueous system (this could effectively decrease the NO3 levels of our systems). [10]
How Do We Grow Them?
In nature, the deep crevices and overhangs provide ample room for sponges to fulfill the environmental niches I discussed above. Our glass boxes fail to provide an ample environment for sponges to grow to a high enough density to reap the benefits of sponge looping. My proposition – let’s go old school and embrace cryptic sumps.
Cryptic sumps are a dedicated area in a system that allows for a combination of high turnover rate, and close to zero exposure to a light source. Lack of light prevents the proliferation of any photosynthetic organisms. At the same time, a high turnover rate allows filter-feeding benthic creatures to thrive, given sufficient nutrient input. This technique was created in 1996/1997 and popularized in the early 2000s by Steven Tyree, who championed filtration systems built on natural organisms such as sponges and sea squirts instead of aggressive mechanical filtration. [11]
If you have an existing system, an easy way to add a cryptic sump would be to add a canister filter. One could easily remove all of the media slots and instead add rubble or even whole sponges that could be purchased. If one had a cryptic space then you could theoretically dose silicates to upregulate the growth of sponges and reduce labile DOC at a more rapid rate (a fun experiment for someone to try).

In addition to the positive effects of nutrient absorption, cryptic sumps could also likely provide balance to our microbiomes. In recent years, popular YouTuber “ReefBum ” posted a video showcasing his experience plumbing in a cryptic sump. He shared many positive observations, but more importantly, he also shared Aquabiomics reports taken before and after adding a cryptic zone.
After installing a cryptic sump, his bacterial diversity score increased by 139 points, and his bacterial balance score increased by 64. When analyzing his community composition, the most striking thing to me was a significant decrease in Vibrionacea and an increase in Pelagibacteracea. This shift could have occurred due to a corresponding change in DOC as Vibrio tends to grow in environments with high DOC and Pelagibacter in environments with low DOC. [12]
How Can DOC Be Monitored?
The most straightforward way to monitor the organic carbon in one’s system is to send in a Triton N-DOC test. They are the only company on the market that offers this service. Due to everything discussed in this article, monitoring DOC may be the most critical value to chase above pH and traces.
In my mind, a system that’s stable with an average pH of 7.8 and a few minor traces missing with low DOC would likely be much healthier than a system where pH is chased to 8.5 and has perfect ICP results but a high DOC value. Based on available data, DOC is the most significant determining factor for microbiome structure (assuming other core parameters are stable). Thus, if one’s DOC is too high, their microbiome is likely out of wack, and consequently, they have little to no buffer zone for any swing.
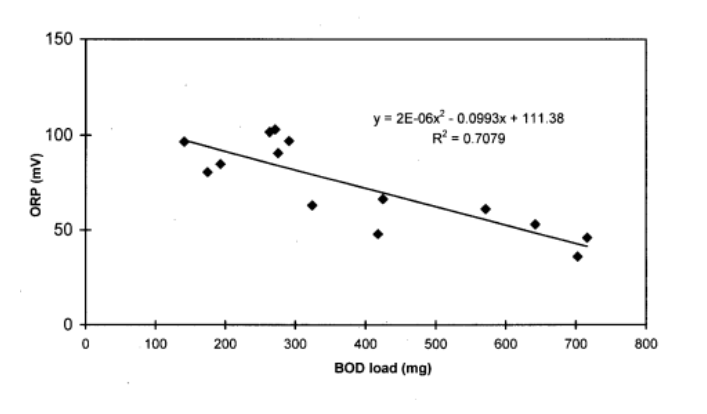
Another potential way to monitor DOC at a moment’s notice is to utilize ORP. I looked for hours and could not find any papers that directly correlated ORP to the TOC or DOC of a system. However, extensive data illustrates that ORP negatively correlates with biochemical oxygen demand (BOD). It’s also well established that BOD values have a very close association with the TOC of a system. Thus, a logical extension can be made that because BOD = TOC and BOD is inverse to ORP, this must mean that ORP is inverse to TOC. [13]
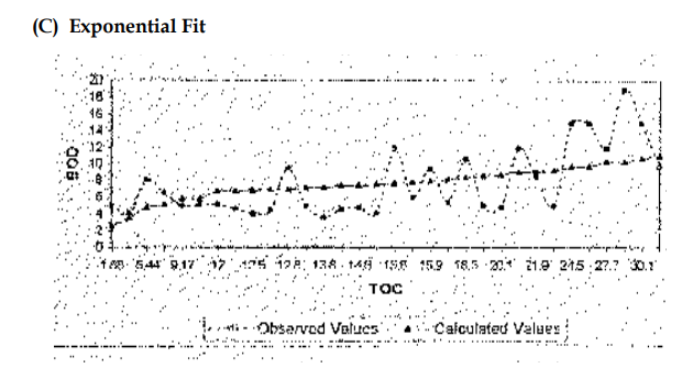
Thus, if one has a low ORP value, that could indicate high TOC/DOC (in some instances) since ORP measures the total proportion of oxidizers and reducers, many other variables could lead to a low ORP value. On average, though, since TOC measures the total organic carbon in a system and organic molecules consume oxidizing agents, one of the primary culprits of lower ORP values in our systems is high TOC/DOC.
If someone can access a total organic carbon analyzer, it would be great to generate data in a controlled system to establish a clear statistical trend between ORP and TOC. In no way is an ORP probe a replacement for an N-DOC test, but it could be an excellent tool to utilize at a moment’s notice to “guesstimate” the TOC values of our systems. [14]
Now that we have discussed what issues high DOC can cause, how to test for it, and how to manage it, it’s essential to identify how these strategies can mesh with existing methods. The easiest way to resolve the issues associated with DOC and the microbiome is to embrace a new method of managing our systems that puts them at the top of our priority list. This method would require embracing recent years’ organic chemistry and microbiology tools.
Ultimately we need a method that effectively synthesizes these advances with the inorganic stability that’s been achieved by automation and ICP testing. This would finally give us a bird’s eye view of the biochemical systems our tanks represent taking into account how organic and inorganic systems ultimately interact with biological systems.
In the remainder of this article, I will lay the foundation of what such an approach entails by describing seven primary principles that should inform our methods as an industry moving forward.
Tying Things Up: A Holistic Method To Reefing
1. Don’t Dose What You Don’t Test For
It’s incredibly ironic that many people whose opinions form the foundation of this industry both simultaneously praise this moniker and seemingly cherry-pick its deployment to justify their misguided practices. The most obvious example of this is carbon dosing and adding bacterial supplements.
Many swear by certain foods, certain bottles of microbes, or a specific carbon source despite never testing what’s in these products or how their addition may affect their systems as a function of time. Whenever I have heard these community members met with the question, “Have you tested your microbiome?” it’s often met with dismay and annoyance about how the results mean nothing, etc.
This highlights a fundamental contraction held amongst the industry’s thought leaders. There’s a solid investment in the idea of “if it isn’t broken, don’t fix it.” This belief is the death knell of innovation. The overemphasis of experiential knowledge and anecdote combined with tribalism and the fear of change has led to the attachment of ideas which may be best left in the past. This has created an environment of stagnation. Plain and simple – it’s time to move on and push forward. We must simultaneously evaluate the old and new.
In a hobby where we spend thousands of dollars to keep our animals alive, we should settle for nothing less than the best. In terms of the current methods and practices in the industry we are being promised Michelin star quality but yet we continue to get food poisoning.
We’re all invested in the equivalent of Schrodinger’s Coral – there’s an equal probability that when you view your glass box every morning, your animals may be dead or alive. The only way to increase our animals’ likelihood of survival is to determine the best methods to care for them. Nearly every other industry focused on animal care devotes copious amounts of time and research to assess their methods.
The reptile industry has determined what spectrum of UV reptiles utilize most efficiently and they then tailor their products to the animal. Zoos and public aquariums constantly track weight and caloric intake and adjust the diet plans of their animals based on how they’re responding and growing. The reef industry? We listen to the same ten people who have been driving innovation since the early 2000s and take what they say as the gold standard due to their experience.
Just because you’ve done something for 25 years doesn’t mean you’ve done it right. Anecdotal observation and conjecture can only take us so far. I applaud all the trailblazers who have spent time and energy determining the baseline methods we use to keep corals. But it’s now time to narrow down the best methods for our animals. It’s time we put our animals above our egos.
2. Filter Decisions Through the Microbiome
As discussed above, ozone could potentially be a valuable tool in managing DOC as it could theoretically oxidize organics to a state where they’re more easily removed by other filtration methods. The issue is ozone also likely hurts the microbiome. Thus, this is a tool I would never add to my system.
It’s essential to understand the health of the entire system depends on the microbial community. The planktonic community of the system and the localized community on and in the corals are linked as the planktonic community holds both picoplankton, which make up the primary heterotrophic food source of corals, as well as species that can act as potential pathogens.
We are fundamentally fighting an uphill battle if we deploy a tool such as a UV sterilizer or ozone or start our system with dry rock and bottled bacteria. Our systems are likely to have low diversity and lack many core bacterial families that help the long-term health of our corals by providing them food, metabolizing info chemicals, and directly competing with external pathogens. In short, if we keep our microbiome happy, all our animals will be satisfied.
We are fundamentally all biome keepers – we all understand the importance of cultivating nitrifying bacteria and cycling our systems. Now that we can test our systems and see what microbes reside in them, we should keep the same vigor we have with the microbiome at the start of our systems and continue to observe and attempt to manage our microbiome over the life of our system just as we would any other parameter we can test for.
3. Chase DOC
Until we can determine what approach can manage DOC most effectively without any negative downside, trailblazers should likely send out N-DOC tests monthly and attempt to tinker and evaluate what methods work best for their system. As discussed above, cryptic sumps represent our best tool in DOC management. They have little to no downsides and offer many other benefits, such as microbial stability and phosphate reduction. The combination of GAC and a good skimmer could manage things well, but there may be downsides to using GAC.
I would like to see data comparing a system with skimmer + GAC vs a system with skimmer + cryptic sump in DOC export. For those unwilling to tinker with their systems, the easiest way to manage DOC is to have a regular water change schedule. So long as your nutrients are within range and your system is stable, conducting 10-20{4213dd704bcac8bc0f4b7ed263c80e178fe24d9d0d3162a98d050d1d839166e8} water changes with a high-quality salt could go a long way in keeping DOC levels down.
Those who no longer do water changes are most likely to encounter DOC-related issues. Therefore, if you do water changes on your system and you’re having problems with your system, it’s less likely that those issues stem from high DOC. Even so, evaluating your system’s DOC to establish a baseline is a good idea.
4. Proper Nutrition
Another critical aspect of managing DOC is to rethink how it’s introduced to a system. During this analysis, we must focus on our current nutrient import strategies. While data is needed on this question, I suspect that refugiums, the use of amino acids, and the current formulation of most coral foods are best left in the past. These approaches could be replaced with cryptic sumps, live phytoplankton, and the addition of live bacterioplankton.
The use of live foods would help to manage excess nitrogen and phosphorus in the system and provide both the protein, amino acid, and fatty acid sources corals for nutrition in a targeted way compared to the shotgun approach of adding in every amino acid and some yeast and fish extract along with random strains of terrestrial bacteria and hoping for the best.
Replacing the complex mixture of food currently on the market, which typically introduces excess N, P, DOC, or bacterial strains, with the species of zoo, phyto, and pico plankton that corals are proven to eat would allow for systems that are lockstep with natural reefs. It’s important to understand that corals are mixotrophs capable of meeting their nutritional needs from many sources. They can get energy via photosynthesis from their zooxanthellae, actively absorb inorganic nitrogen and phosphorus through their tissue, or capture prey items to eat.
In nature, corals rely primarily on photosynthesis, then heterotrophy and a small part of their metabolism comes from the nutrients they absorb. In our systems, because we don’t give corals the heterotrophic food they have evolved to depend on, we supplement their nutritional needs by keeping our inorganic nutrient values much higher than those of natural reefs.
If coral’s dietary needs are met via heterotrophy, they need to absorb very little inorganic N and P. Thus, in the future, as products containing bacterioplankton hopefully begin to enter the market, this could allow us to keep our tanks with low nutrient values.
Keeping our inorganic nutrients at near undetectable levels would aid in curbing external algae growth (and the introduction of DOC it brings) and also shift the microbiome to a more oligotrophic state, which would favor the growth of beneficial species and downregulate the growth of pathogenic ones.
Another aspect to consider with inorganic nutrition is that corals can only utilize ammonia as a nitrogen source. Substantial evidence shows that corals that receive ammonia in high DOC environments are much more likely to survive than those that don’t.
It’s been illustrated that high DOC values deplete corals’ internal nitrogen reserves; thus, providing them with an external nitrogen source they selectively metabolize can help maintain their health even in adverse conditions. Based on the available data, nitrates are likely consumed by opportunistic bacteria, not coral. [15]
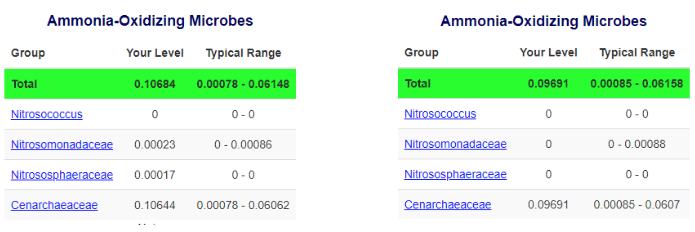
On the flip side, Dr. Andrew Bouwma recently conducted an Aquabiomics test before and after dosing ammonia and observed explosive growth in his corals and no shift in the microbiome (even among ammonia-oxidizing families). This helps provide further evidence that corals absorb ammonia so rapidly that no microbes can metabolize it. [16]
The approach of adding live food, ammonia, and phosphorous as the primary forms of nutrient input and then utilizing a skimmer and cryptic sump as the method of nutrient output would mimic natural ecosystems where there are large upwellings of plankton of all trophic levels and removal of excess nutrients via sponge looping and fractionation. This provides a much more surgical approach to import-export based on data from current literature.
5. Stability
I put this lower on the list since, as of now, we do a reasonably good job at keeping the inorganic chemistry of our systems stable with automation and ICP testing. Maintaining this stability is essential – it should just be expanded into the realm of organic chemistry. Keeping our parameters stable once in the ideal range is critical to avoid the exact differential mechanism discussed earlier that causes good bacteria to become bad. The more stable our systems are, the less likely pathogenic events will occur.
6. Mimic Nature
Inherently, this approach is based on modeling natural environments. Such mimicry should include:
- Striving for oligotrophic conditions and heavy feeding of live foods.
- Stability.
- Full spectrum lighting.
- High laminar flow.
- Keeping the right proportion of herbivores in a system.
- Maintaining the system in a high oxygen state.
- Only adding microbes endemic to marine environments.
- Supplying the trace elements corals are proven to utilize for natural growth.
7. Skepticism
Many of the products on the market are built on a foundation of sand with little to no data supporting their use. For far too long, the companies in this industry have failed to provide answers. Now that we can arm ourselves with data, we should band together and discover the truth ourselves. Don’t be satisfied with the current narrative – help push the boundaries of this industry by responsibly experimenting with your system.
We now have the technology to evaluate our systems’ microbiome and limited organic chemistry questions. Utilizing these new approaches, we should systematically test the effects of every practice we have taken as “true.” “Things looked better” or “I’ve been doing this for 15 years” are no longer good enough justifications to support an argument. We need a combination of qualitative and quantitative data.
There’s Much More Work To Be Done
While the core concepts I spoke about today are rooted in years of data, their application to our systems is primarily a logical extension of mine. Thus, don’t take this article as a recommendation to stop utilizing a refugium, carbon dosing, feeding, etc. Consider it as a skeptical evaluation of our practices and a suggestion for experimentation. Since people can purchase N-DOC tests, many of the questions I posed in this article can be answered in a community science fashion.
Starting in July, Reef Builders will analyze the effect of carbon dosing on our systems’ microbiome. I hypothesize that carbon dosing, regardless of the carbon source, will lead to an imbalance in the microbiome and promote pathogenic families due to the introduction of excess DOC.
By the end of August, we will determine whether my hypothesis is correct. In the coming years, Reef Builders hopes to organize many more community science projects so we can finally answer what methods work best and which to leave in the past.
Enough smoke and mirrors – it’s time that we, as Reef Builders, fundamentally rebuild the foundation of how we reef.
References
[1] Kuntz, N. M., Kline, D. I., Sandin, S. A., & Rohwer, F. (2005, June 9). Pathologies and mortality rates caused by organic carbon and nutrient stressors in three Caribbean coral species. Marine Ecology Progress Series. https://www.int-res.com/abstracts/meps/v294/p173-180/
[2] Haas, A. F., Fairoz, M. F., Kelly, L. W., Nelson, C. E., Dinsdale, E. A., Edwards, R. A., Giles, S., Hatay, M., Hisakawa, N., Knowles, B., Lim, Y. W., Maughan, H., Pantos, O., Roach, T. N., Sanchez, S. E., Silveira, C. B., Sandin, S., Smith, J. E., & Rohwer, F. (2016). Global microbialization of coral reefs. Nature Microbiology, 1(6), 1-7. https://doi.org/10.1038/nmicrobiol.2016.42
[3] Vega Thurber, R., Willner-Hall, D., Rodriguez-Mueller, B., Desnues, C., . Edwards, R. A., Angly, F., Dinsdale, E., Kelly, L., & Rohwer, F. (2009). Metagenomic analysis of stressed coral holobionts. Environmental Microbiology. https://pubmed.ncbi.nlm.nih.gov/19397678/
[4] Cárdenas, A., Neave, M. J., Haroon, M. F., Pogoreutz, C., Rädecker, N., Wild, C., Gärdes, A., & Voolstra, C. R. (2017, September 12). Excess labile carbon promotes the expression of virulence factors in coral reef bacterioplankton. Nature News. https://www.nature.com/articles/ismej2017142
[5] Hoadley, K. D., Hamilton, M., Poirier, C. L., Choi, C. J., Yung, M., & Worden, A. Z. (2021). Selective Uptake of Pelagic Microbial Community Members by Caribbean Reef Corals. Applied and Environmental Microbiology, 87(9). https://doi.org/10.1128/AEM.03175-20
[6] Giovannoni, S. J. (2017, January 3). Sar11 bacteria: The most abundant plankton in the oceans. Annual Review of Marine Science. https://www.annualreviews.org/content/journals/10.1146/annurev-marine-010814-015934
[7] Feldman, K. S. (2010, February 15). Elemental analysis of skimmate: What does a protein skimmer actually remove from aquarium water?. Reefs.com. https://reefs.com/magazine/elemental-analysis-of-skimmate-what-does-a-protein-skimmer-actually-remove-from-aquarium-water/
[8] Hatt, J., Germain-Cripps, E., & Judd, S. jon. (2013, January). Granular activated carbon for removal of organic matter and turbidity from secondary wastewater. Research Gate. https://www.researchgate.net/publication/234104397_Granular_activated_carbon_for_removal_of_organic_matter_and_turbidity_from_secondary_wastewater
[9] Jasper Merijn, de G. (2009). Element cycling on tropical coral reefs. University of Groningen. https://pure.rug.nl/ws/portalfiles/portal/14555023/01c1.pdf
[10] Pawlik, J. R., & McMurray, S. E. (2019, June 21). The emerging ecological and biogeochemical importance … – annual reviews. The Emerging Ecological and Biogeochemical Importance of Sponges on Coral Reefs. https://www.annualreviews.org/…/annurev-marine-010419…
[11] Tyree, S. (n.d.). Captive Maintenance Advanced Techniques – Volume 2 The Cryptic Zonal System. Reeffarmers.com. http://www.reeffarmers.com/newbook.htm
[12] [ReefBum]. (2022, December 5). “Cryptic Sump – The Data is In. Is it Effective?” [Video]. https://www.youtube.com/watch?v=IbCm2pVeIv8
[13] Ramanand Bhat, M., Roopali, Hiremath, S., & . Kulkarni, V. R. (2003). Correlation between BOD, COD and TOC. https://www.researchgate.net/profile/Anoop-Srivastava/post/What_is_the_relationship_between_dissolved_organic_matter_with_BOD_COD_TOC_of_wastewater/attachment/59d6519379197b80779aa0f2/AS:507962524475392@1498119111147/download/correlation-between-bod-cod-and-toc-.pdf
[14] Ra, C.-S. (1997, January 1). Oxidation reduction potential (ORP) as a real-time control parameter in swine manure treatment process. Open Collections. https://open.library.ubc.ca/soa/cIRcle/collections/ubctheses/831/items/1.0058607
[15] Bednarz, V. N., Grover, R., & Ferrier-Pagès, C. (2020). Elevated ammonium delays the impairment of the coral-dinoflagellate symbiosis during labile carbon pollution. Aquatic Toxicology, 218, 105360. https://doi.org/10.1016/j.aquatox.2019.105360
[16] [ReefBum]. (2024, April 23). Rappin’ with reefbum: Guests dr. Eli Meyer, AquaBiomics/dr. Andrew Bouwma, science educator/hobbyist. YouTube. https://www.youtube.com/watch?v=i0P3bWcK72w
Other Resources Used For This Article
Becker, C. C., Weber, L., Zgliczynski, B., Sullivan, C., Sandin, S., Muller, E., Clark, A. S., Kido Soule, M. C., Longnecker, K., Kujawinski, E. B., & Apprill, A. (2023, September 5). Microorganisms and dissolved metabolites distinguish Florida’s coral reef habitats. OUP Academic. https://academic.oup.com/pnasnexus/article/2/9/pgad287/7259987



